Visionary Clinical Toxicologist Gwen McMillin Has Transformed Drug Testing to Better Support Clinical Needs

Colleagues describe Gwen McMillin, PhD, DABCC (CC, TC), FAACC, scientific director for the mass spectrometry platform and medical director of Clinical Toxicology at ARUP Laboratories, as someone who has a vast and intricate knowledge of everything related to clinical toxicology but who never loses sight of the goal of testing: to empower clinicians with knowledge to aid their patients.
“She’s always thinking about how these test results will be used at the end of the day. The nitty-gritty details of the testing, the processes, the reporting are important, but she never forgets to consider the clinical impact,” said Joely Straseski, PhD, MS, MT(ASCP), DABCC, FAACC, section chief of Chemistry and medical director of Endocrinology at ARUP.
That unique awareness is evident in the various ways McMillin has transformed the field of clinical toxicology, pushing testing to become more relevant and more efficient and finding new solutions to challenging problems.
McMillin was instrumental in shifting the field from a standard drug testing model that used both a screening and a confirmatory test for every drug test. The standard screening test, an immunoassay, often fails to adequately identify compounds. McMillin advocated instead for an approach that would skip the screening immunoassay and go straight to the more accurate testing method when indicated by clinical situation. The new approach reduces unnecessary testing, yields faster results, and provides more useful information to better inform treatment decisions.
“We have been leaders in the philosophy that you don’t need to follow the traditional ‘screen with reflex to confirmation’ approach for clinical toxicology. It makes more sense to align the test with the clinical purpose and receive the result you need up front, rather than going through a series of tests,” McMillin said.
Although McMillin’s approach initially met with resistance, her extensive efforts to educate clinicians and advocate for more effective testing have eventually led many to adopt her approach and to a worldwide recognition of McMillin’s expertise.
She has since contributed to the development of guidelines for the Clinical and Laboratory Standards Institute (CLSI), an organization that publishes recommendations and standards for laboratory testing.
McMillin’s expertise is respected not only within the laboratory community—she’s also frequently called upon as an expert witness in court proceedings. Recently, McMillin was asked to provide her expertise in a case in which a woman had been accused of resuming meth use based on the results of an immunoassay screen.
“Women who are pregnant are often treated with a medication called labetalol due to their high blood pressure during pregnancy,” McMillin said. “Labetalol will cause a false positive for methamphetamine because of its chemistry, which is similar to that of methamphetamine.”
McMillin’s testimony clarified the limitations of immunoassay screening and its potential to provide false-positive results and demonstrated that an immunoassay alone was insufficient evidence to prove the patient had returned to meth use.
“Gwen’s expertise in test interpretation has had a profound impact in so many aspects of clinical care. She’s a shining example of being able to align a clinical focus with research and test development, and she’s had a strong influence on our Clinical Toxicology Laboratory,” said Jonathan R. Genzen, MD, PhD, ARUP chief medical officer.
The Road to Renown
McMillin’s interest in clinical toxicology stems from a personal experience in which she was able to closely observe the differing effects of a variety of medications.
As a teenager, McMillin watched her youngest brother suffer with a seizure disorder. More than ten years his senior, she took an active role in his care, helping to administer medications and record his seizure types.
“It was horrific to witness his suffering, but also fascinating to me to observe how the different medications affected him—which ones worked, which ones didn’t, and how they interacted with each other,” McMillin said.
Fortunately, her brother eventually outgrew the condition, but the experience spurred McMillin to study neuropharmacology during her undergraduate education at Grinnell College.
During graduate school, McMillin studied pharmacology and focused her efforts on anticonvulsant drug development and mechanistic studies. As part of her first faculty position in the College of Pharmacy at the University of Utah, McMillin led the mechanistic studies for the U’s Anticonvulsant Drug Development Program. The project evaluated drugs for their efficacy in preventing convulsions and developed new antiepileptic agents.
During her studies, McMillin witnessed the harmful effects of the opioid epidemic as a volunteer at the University of Utah Hospital. There, she saw many drug overdoses and drug-related adverse events.
“People were struggling with the very drugs that were supposed to help them, and they didn’t help them. I witnessed therapeutic failure, which I very much considered to be an adverse drug reaction,” McMillin said.
Her experience at the hospital deepened her interest in drug testing. In November 1996, McMillin joined ARUP Laboratories, initially securing a position as a research scientist in the Research Institute. At the time, former ARUP President and CEO Edward Ashwood, MD, recognized her talents and capabilities and encouraged her to pursue becoming a medical director. McMillin returned to the University of Utah to complete a postdoctoral fellowship at the University of Utah School of Medicine.
Focus on Maternal and Pediatric Health
Since joining ARUP, McMillin has continued to drive innovation in clinical toxicology to find better testing solutions that improve patient outcomes.
“My mantra is to take what I’ve learned and turn it into something that is hopefully helpful to everyone,” McMillin said.
McMillin has focused much of her work on supporting maternal and pediatric health needs. As a result of the opioid epidemic, an increase in newborns experiencing neonatal abstinence syndrome generated a need for methods to assess in utero exposure to drugs. McMillin spent five years helping to develop and validate a panel of mass spectrometry newborn drug tests using meconium, a newborn’s first stool.
“I tried to optimize the testing to align better with our clients’ needs by understanding how results were being used, both clinically and in court, and whether that leads us to provide better resources for the mother and family, or to find a better circumstance for the baby,” McMillin said.
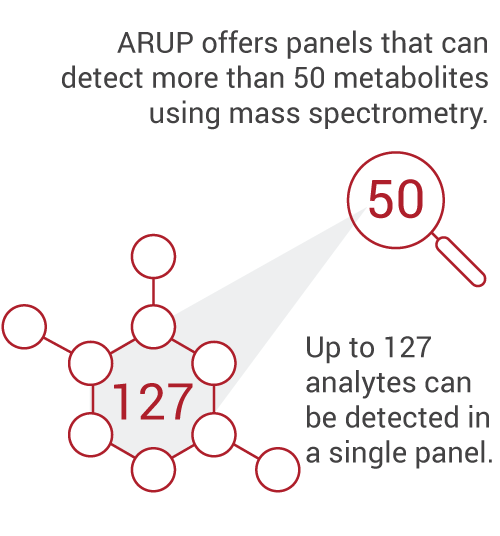
Eventually, she recognized the logistical difficulties of using meconium and explored the possibility of using a sample of umbilical cord tissue instead. As a result of McMillin’s extensive research on the results of umbilical cord tissue testing, she has led ARUP in developing a mass spectrometry test, using umbilical cord tissue as the specimen type, that detects almost 50 different compounds.
“It’s imperative that we have testing to assess in utero drug exposure, as individuals who are pregnant are also vulnerable to substance use disorders,” said Kamisha Johnson-Davis, PhD, DABCC (CC, TC), medical director of Clinical Toxicology. “Gwen has been instrumental in bringing on testing that supports the needs of newborns.”
As part of one of her many research projects, McMillin has collaborated with Torri Metz, MD, MS, an associate professor of Obstetrics and Gynecology and vice chair of research of Obstetrics and Gynecology at the University of Utah, to determine the prevalence of drug use in Utah.
“The data from the cord sampling project have enabled us to help patients in regions that have very high rates of substance use during pregnancy,” Metz said. “The data we collected were used to apply for a grant to establish an entire center for reduction of morbidity and mortality from substance use disorders in pregnancy in Utah.”
The University of Utah has received a $14 million dollar grant to establish the ELEVATE Center: Reduction of Maternal Morbidity from Substance Use Disorder in Utah, which will provide interventions to patients affected by substance use disorders. These interventions include community engagement, clinician and patient education, and ongoing surveillance of the prevalence of the disorders.
“Personally, I find it very satisfying that we can help families get the resources they need; we want to identify newborns exposed to drugs so they can get treated appropriately, but it’s about the downstream effects to make sure that families are put on the best path forward,” McMillin said.
According to Metz, “McMillin has expertise across the entire spectrum, both clinical and research, and her perspective is valuable not only as an expert in lab science, but she also has a sense of what our work means for patients outside of the lab, and how it will trickle down to public health and broader outcomes.”
A Supportive Colleague
McMillin’s near colleagues value her renowned expertise and describe her as incredibly supportive.
“She knows so much and has had such an impact on everything in all of the toxicology labs, and she’s a very supportive colleague,” said Jessica Boyd, PhD, FCACB, DABCC (TC), a fellow medical director of Clinical Toxicology at ARUP.
Vrajesh K. Pandya, PhD, DABCC, medical director of Clinical Chemistry and Toxicology at the University of Utah Hospital Lab, completed a fellowship at ARUP Laboratories during which he collaborated with McMillin on further studies to compare meconium and umbilical cord test results. Pandya described McMillin as a “very proactive and engaged mentor who always roots for your success.”
After Pandya’s transition from fellow to medical director, McMillin was the first person to reach out to acknowledge him as a fellow colleague.
“She’s truly the pioneer of toxicology testing at ARUP. She’s a leading authority in the world on neonatal drug testing as well as pharmacogenetic testing, which is clearly reflected in her publications over the years,” Pandya said. “With Gwen’s leadership in that area and institutional support, ARUP is ready to take on the challenge of the next generation of toxicology testing.”






 Clinical toxicology
Clinical toxicology Chemistry
Chemistry Biochemical genetics
Biochemical genetics